To recover your password please fill in your email address
Please fill in below form to create an account with us

About us
We work closely with clinicians to design studies to assess the performance of tests and testing strategies to improve clinical practice and patient outcomes. We also aim to improve the methods and interpretation of clinical studies of tests, including biomarkers proposed for clinical use.
Work
We have expertise in designing and conducting:
Evidence integration in test research
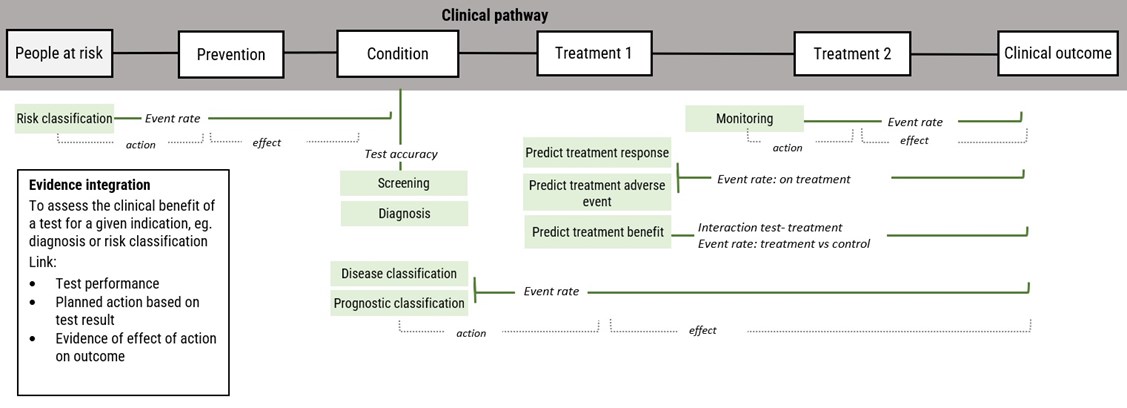
Test development and evaluation typically requires an evidence integration approach. Using this approach, evidence of test performance for a given indication is linked with other types of evidence to assess clinical outcomes. Additional evidence for interpretation of clinical performance measures eg, test accuracy includes the prevalence of the condition, the clinical benefits of actions based on the test result, and potential harms if misclassification occurs.
Studies for test development and regulatory approval: The indication for testing is defined by describing the clinical decision the test is intended to inform and potential clinical benefit for the patient, or implications for patient counselling. This information is used to design analytical and clinical performance studies. Study results are used to assess if the test is fit for purpose as required for regulatory approval.
Studies to inform clinical guidelines: When the test is already used in practice, clinical performance studies can improve interpretation of results including: the prognostic significance of the test results for disease classification (alone or in combination with other tests); and variation in performance in different settings and patient groups. This evidence can inform clinical guidelines for its use.
There are no ‘one size fits all’ rules for minimum acceptable clinical performance. For conditions where there is effective treatment, but no or poor existing tests, a new test with modest accuracy will be valuable to improve health outcomes. For conditions where existing tests perform well and the consequences of misclassification are serious, a new test would need to offer near perfect accuracy.
Funding and collaborators
Clinical researchers, industry, foundations, universities, and government.
Contact us
Medical test research lead Dr Sally Lord: sally.lord@sydney.edu.au
Figure Clinical performance measures for test evaluation and evidence integration for interpretation of clinical benefits
Test indications are shaded green with corresponding clinical performance measures (test accuracy, event rate, interaction test)