To recover your password please fill in your email address
Please fill in below form to create an account with us
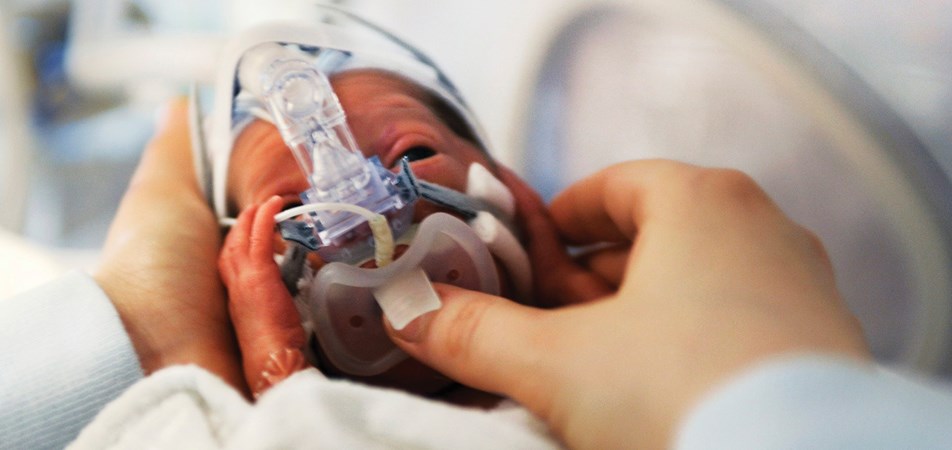
Healthcare consumers (patients) are people who have lived experience of a health issue. They include patients, families and friends, carers and members of the general public.
As part of the CTC’s mission to improve global health outcomes, we engage with consumers around the investigation, design and running of trials to increase a trial’s likelihood of making a positive impact on consumers and the health care system in a number of ways:
Don't just take from us, learn from us
Parents of premature infants and consumer engagement advocates, Melinda Cruz Turner and Natalie Merida speak about the importance of consumer involvement in clinical trials at the Perinatal Society of ANZ Congress in June 2022.
Recruiting patients for trials
The CTC does not recruit participants to clinical trials directly. Trials are usually run through hospitals and other health service providers. To find out more about a trial or whether you can participate in one, please discuss this with your doctor. Comprehensive general information about clinical trials in Australia is available from www.australianclinicaltrials.gov.au. Trials being conducted in Australia can be found on the Australian New Zealand Clinical Trials Registry website. Not all of these trials are currently recruiting patients.