To recover your password please fill in your email address
Please fill in below form to create an account with us

PROJECT OVERVIEW
HeSANDA is a national initiative of the Australian Research Data Commons (ARDC) that makes health and medical research data easier to find. It facilitates access, sharing and reuse of research data, resulting in a reduction in research waste, improvements in researcher collaboration, and an opportunity to answer new research questions. It aims to support more efficient and effective research to help improve health outcomes.
The research community across Australia has been working together through HeSANDA to develop Health Data Australia, a catalogue for health and medical researchers to register a description of their research so it’s easy to discover. By leveraging existing data and leaving the control in custodians’ hands, HeSANDA supports more efficient and effective research to help improve health outcomes.
In Phase 1, HeSANDA focussed on investigator-initiated and academic clinical trials. Phase 2 (2023-2028) will provide opportunities to consolidate capability for clinical trials, extend the approach to other health study types, and deploy new capabilities such as secure access environments.

COLLABORATORS
To date, nine nodes (representing 72 research organisations) have been approved to be involved in the development of the HeSANDA initiative by ARDC including the Sydney Node (short for Sydney Health Partners and NHMRC Clinical Trials Centre Node). Other key collaborators from the ANZCTR, the Australian Institute of Health and Welfare, the Australian Clinical Trials Alliance, NHMRC, Cochrane Australia, Research Australia, and the Population Health Research Network are also contributing to this initiative.
HOW HeSANDA WORKS
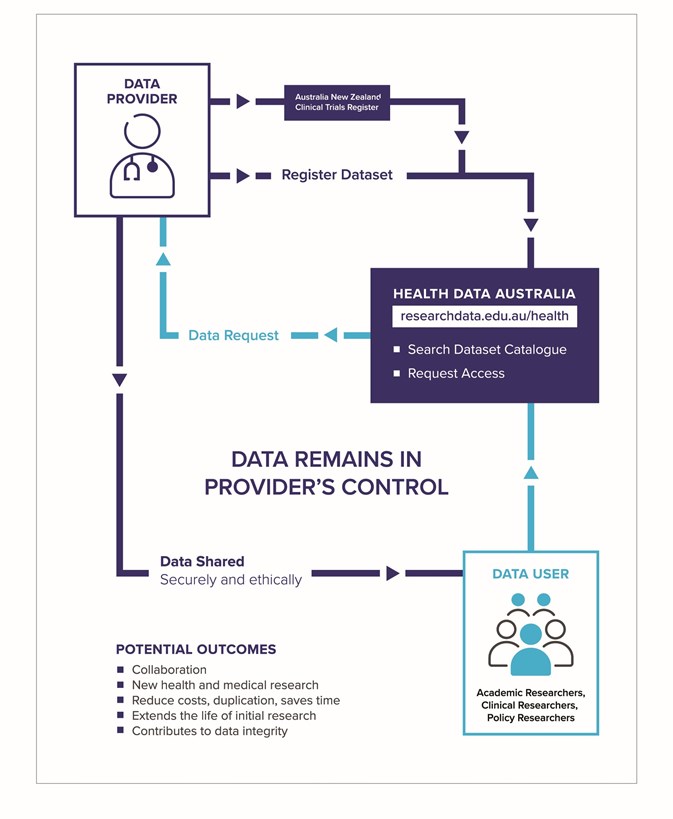
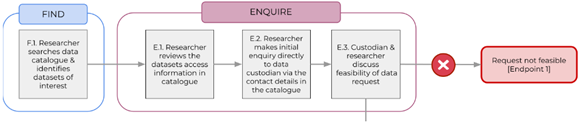
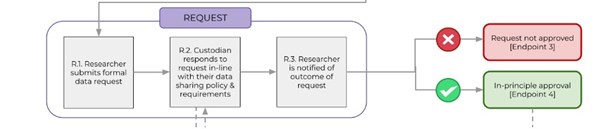
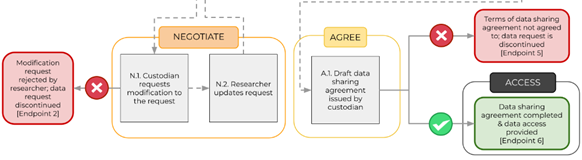
DATA REQUEST WORKFLOW


FUNDING
The Sydney Node of the HeSANDA project received investment from the Australian Research Data Commons. The ARDC is funded by the National Collaborative Research Infrastructure Strategy.
CHIEF INVESTIGATORS
The Sydney Node partners are described below:
|
Organisation |
Partner |
Role in HeSANDA Sydney Node |
|
NHMRC Clinical Trials Centre, University of Sydney |
Angela Webster, Director of Evidence Integration |
Leader |
|
Sydney Health Partners |
Don Nutbeam, Executive Director |
Project oversight |
|
Westmead Applied Research Centre, WSLHD |
Clara Chow, Academic Director |
Data Contributor |
|
Health and Clinical Analytics, University of Sydney |
Thomas Snelling, Director |
Data Contributor |
|
Cardiovascular Centre of Excellence, NSLHD |
Gemma Figtree, Chair of Cardiovascular Initiative |
Data Contributor |
|
Institute for Musculoskeletal Health, University of Sydney |
Chris Maher, Director |
Data Contributor |
|
Institute of Bone and Joint Research, University of Sydney |
David Hunter, Chair |
Data Contributor |
|
Westmead Institute for Medical Research, WSLHD |
Philip O’Connell, Director |
Data Contributor |
|
The Brain and Mind Centre, University of Sydney |
Matthew Kieran, Director |
Data Contributor |
|
Woolcock Institute for Medical Research, University of Sydney |
Bandana Saini, Affiliate Staff (Woolcock) and Director, Academic Education (USyd) |
Data Contributor |
|
Clinical Trials Support Office, Office of the Pro-Vice Chancellor (Research), University of Sydney |
Olya Ryjenko, Clinical Trials Data Lead |
Infrastructure operator |
|
Digital Health CRC |
Tim Shaw, Director |
Infrastructure operator |
|
Information and Communications Technology (ICT), University of Sydney |
Ryan Sullivan, Product Manager |
Infrastructure operator |
|
Sydney Local Health District |
Steve Chadban, Director, Renal Medicine |
Data Contributor |
|
Sydney Local Health District |
Geoff McCaughan, Clinical Director, GE/Liver Services |
Data Contributor |
|
Sydney Local Health District, ICT |
Mitchell Burger, Director, Strategy, Architecture, Innovation and Research |
Infrastructure operator |
KEY LINKS
HOW CAN YOU CONTRIBUTE
To register your clinical trial in Health Data Australia, please complete this form.
CONTACT
For further information please contact hesanda.shp-ctcnode@sydney.edu.au.