To recover your password please fill in your email address
Please fill in below form to create an account with us


 A Randomised, Embedded, Multi-factorial, Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP)
A Randomised, Embedded, Multi-factorial, Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP)
Background
REMAP-CAP is an investigator-initiated, multicentre, international, open label, Bayesian, randomised adaptive platform trial that was established to evaluate multiple aspects of care for patients who are admitted to an Intensive Care Unit (ICU) with severe Community Acquired Pneumonia (CAP) and patients with acute illness due to suspected or proven COVID-19. The platform was designed to adapt during a pandemic to answer time-critical questions related to the effectiveness of a range of intensive care treatments.
In this platform, multiple treatments across domains of therapeutic care are assessed within the same trial. Frequent analyses are undertaken to evaluate the effectiveness of these interventions and participants are randomised until there is sufficient evidence of superiority, inferiority or futility of the intervention. This provides the most efficient structure for evidence generation, whilst pre-specified adaptations in specific domains increase the chances that patients receive the most promising treatment in that domain.
The CTC is responsible for the coordination and management of sites in NSW. The primary project management team is based at the Australian and New Zealand Intensive Care Research Centre, Monash University.
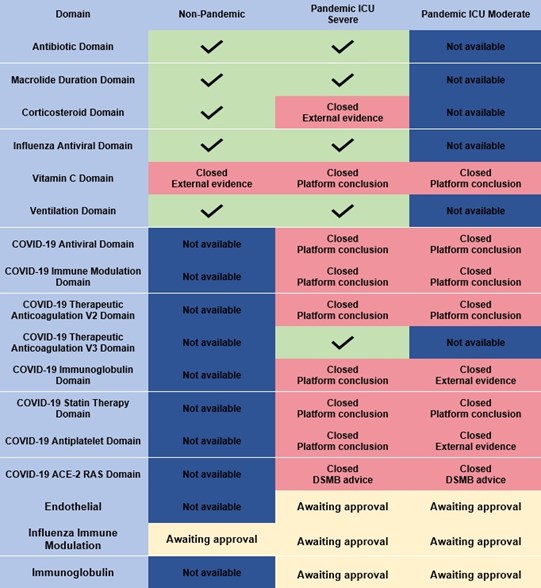
The REMAP-CAP domains are as follows:

|
Aim: |
The primary objective of the REMAP is to identify the effect of a range of interventions to improve outcome for patients admitted to a hospital with community acquired pneumonia including COVID-19 pandemic infection |
|
Supported By: |
National Health Medical Research Council (commencing 2017) Minderoo Foundation (commencing 2020) Office Health Medical Research, NSW Health (commencing 2021) Medical Research Future Fund (commencing 2022) |
|
Eligibility: |
In order to be eligible to participate in the pandemic aspects of REMAP-CAP, a patient must meet the following criteria:
In order to be eligible to participate in the non-pandemic aspects of REMAP-CAP, a patient must meet the following criteria:
|
|
Registration ID: |
ClinicalTrials.gov Identifier: NCT02735707 |
|
Participation: |
International (Australia, Belgium, Canada, Colombia, Croatia, Czech Republic, Estonia, Finland, France, Germany, Hungary, India, Ireland, Israel, Italy, Japan, Kingdom of Saudi Arabia, Malaysia, Nepal, Netherlands, New Zealand, Pakistan, Portugal, Serbia, Singapore, Slovenia, Spain, Switzerland, United Kingdom, United States of America) |
|
Australian Lead Group: |
Australia New Zealand Intensive Care Research Centre (ANZIC-RC), Monash University |
|
Status: |
Active, recruiting |
|
Activation Date: |
2018 – non pandemic recruitment commenced in Australia 2020 – pandemic recruitment commenced in Australia |
|
Chairs: |
David Gattas (Co-chair NSW REMAP-CAP Infrastructure Committee) Manoj Saxena (Co-chair NSW REMAP-CAP Infrastructure Committee) Aidan Burrell (Australian Lead Investigator) Steve Webb (ANZ Executive Director) |
|
Contacts: |
Claire Reynolds | Senior Trial Operations Coordinator Claire.reynolds@sydney.edu.au Jane Parker | REMAP-CAP Project Manager Jane.Parker@monash.edu https://www.remapcap.org/ |
|
Publications: |
|